Once proteins are translated by ribosomes, the residues may be chemically modified such as serine phosphorylation and lysine acetylation. We are interested in how cells modify the proteins and how the PTMs alter the protein functions at molecular level.
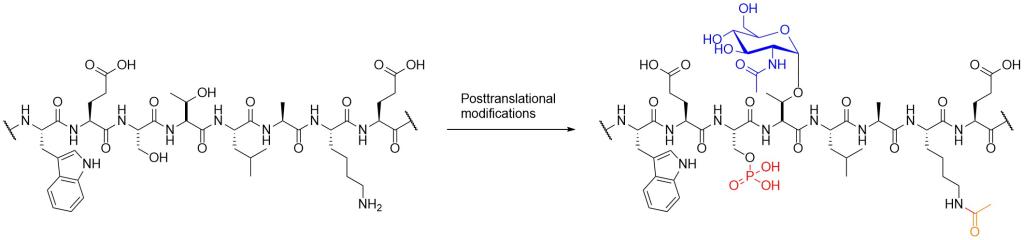
Protein semisynthesis is a chemical strategy to prepare proteins with site specific modifications, that takes advantages of protein expression and peptide synthesis. In order to study mechanisms of PTMs, we are making modified proteins of interest and also developing new chemical methods of protein semisynthesis.

We are developing chemical probes at protein level to study the catalytic mechanisms and protein-protein interactions. Here are two published examples:
Hydroxamic acid-Lys analogs bind HDAC1 (histone deacetylase 1)
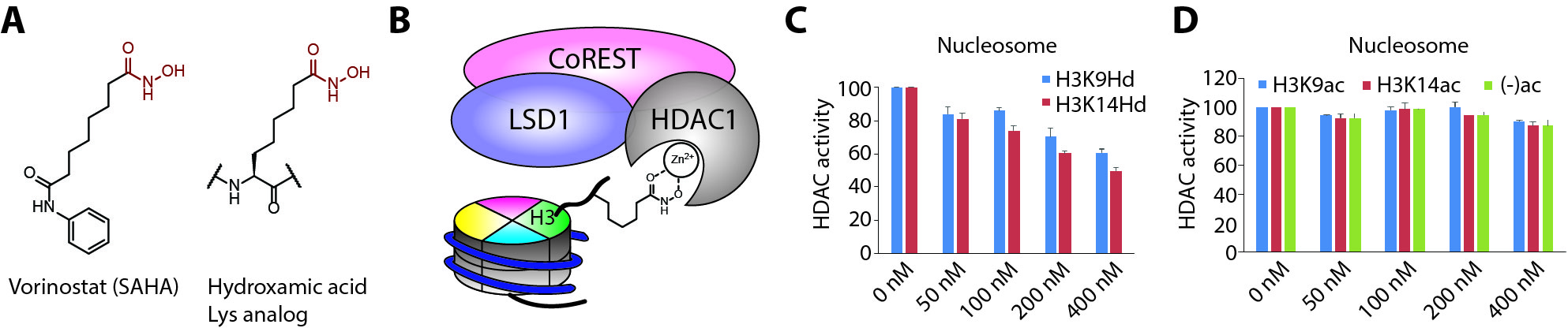
The figure is modified from eLife 2018, 7:e37231
Propargyl-Lys analogs capture LSD1 (lysine-specific demethylase 1)

The figure is modified from Cell Rep. 2020, 30, 2699